New Approaches in Organic Chemistry
1. Catalyst-Free
Oxidation of Sulfides to Sulfoxides by gem-Dihydroperoxide
under Mild Conditions
(SynLett, DOI: 10.1055/a-2015-7526)
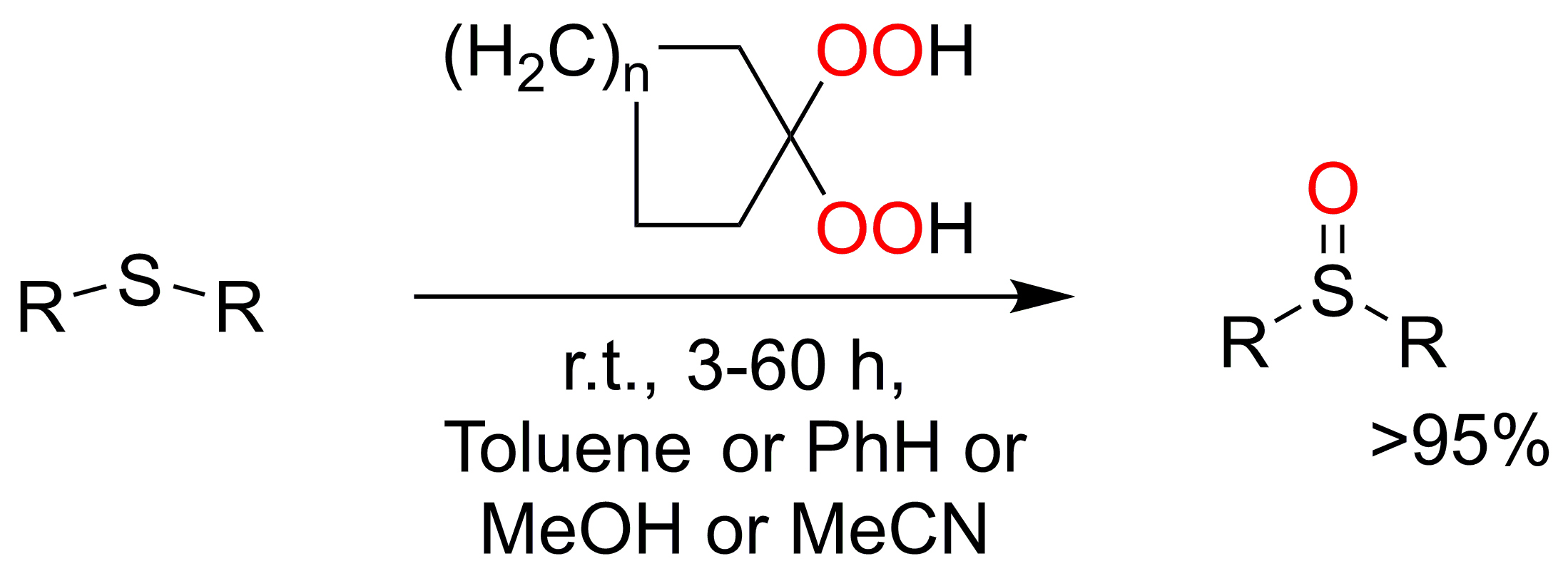
A facile
and efficient method for the oxidation of sulfides (dialkyl,
phenylalkyl, benzylalkyl)
to sulfoxides under mild conditions without using any catalysts is reported. This method afforded a series of sulfoxides
with good yields (>95%). The ready accessibility and low cost of the gem-dihydroperoxides will endow it with attractive applications
in chemical synthesis as oxidants.
2. High-Pressure
Pathway in the Two-Stage Synthesis of 5-Amino-3-Hydroxy-1-Phenyl-1H-Pyrazole
(Lett. Org. Chem., DOI: 10.2174/0115701786331865241120043030)
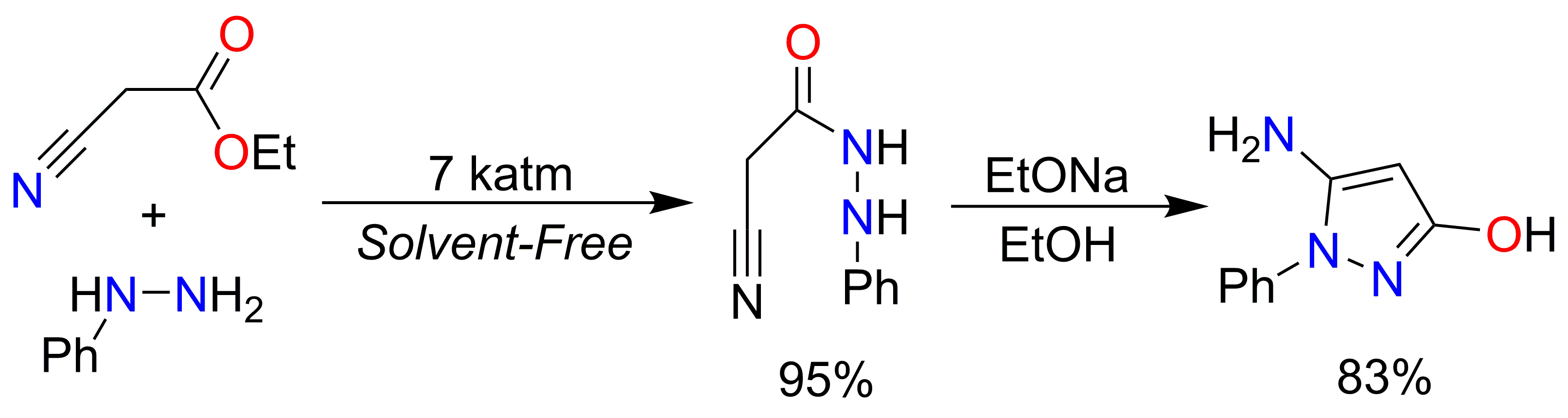
5-Amino-3-hydroxy-1-phenyl-1H-pyrazol
and 3-amino-5-hydroxy-1-phenyl-1H-pyrazol are widely used as synthons in
organic and pharmaceutical chemistry. We developed a high-yield synthesis
method for 5-amino-3-hydroxy-1-phenyl-1H-pyrazol using high-pressure and
base catalysis, achieving up to 80% yield. This method significantly
outperforms existing techniques, which yield no more than 39%. The synthesis
was performed at pressures up to 10 katm, both in
solvent-free conditions and in the presence of solvents, such as methanol,
ethanol, toluene, tert-butyl methyl ether, and
1,4-dioxane. Thermodynamic parameters of possible paths were
calculated using the SMD-M06-2X/MG3S method. Applying high pressure (7 katm) enables the solvent-free and catalyst-free synthesis
of 2-cyano-N'-phenylacetohydrazide with a yield of 96%. This compound can subsequently be converted into
5-amino-3-hydroxy-1-phenyl-1H-pyrazol with yields of up to 90% using
base catalysis. Additionally, the reaction pathways of phenylhydrazine
with ethyl cyanoacetate and its anion have been explored. These pathways are discussed in terms of
thermodynamic potentials calculated using the SMD-M06-2X/MG3S method. High
pressure significantly accelerates the reaction between phenylhydrazine
and ethyl cyanoacetate, leading to the formation of
2-cyano-N'-phenylacetohydrazide. This intermediate can
then be easily converted into 5-amino-3-hydroxy-1-phenyl-1H-pyrazol.
Under neutral conditions, the most favorable reaction pathway involves the
attack of the terminal nitrogen of phenylhydrazine on
the carbonyl group. In the case of the ethyl cyanoacetate
anion, the attack also targets the carbonyl group, but occurs via the
phenyl-substituted nitrogen.
3. Epoxidation of Polyunsaturated Fatty Acid
Double Bonds by Dioxirane Reagent: Regioselectivity and Lipid Supramolecular Organization
(Helvetica Chimica Acta,
DOI: 10.1002/hlca.200690209)
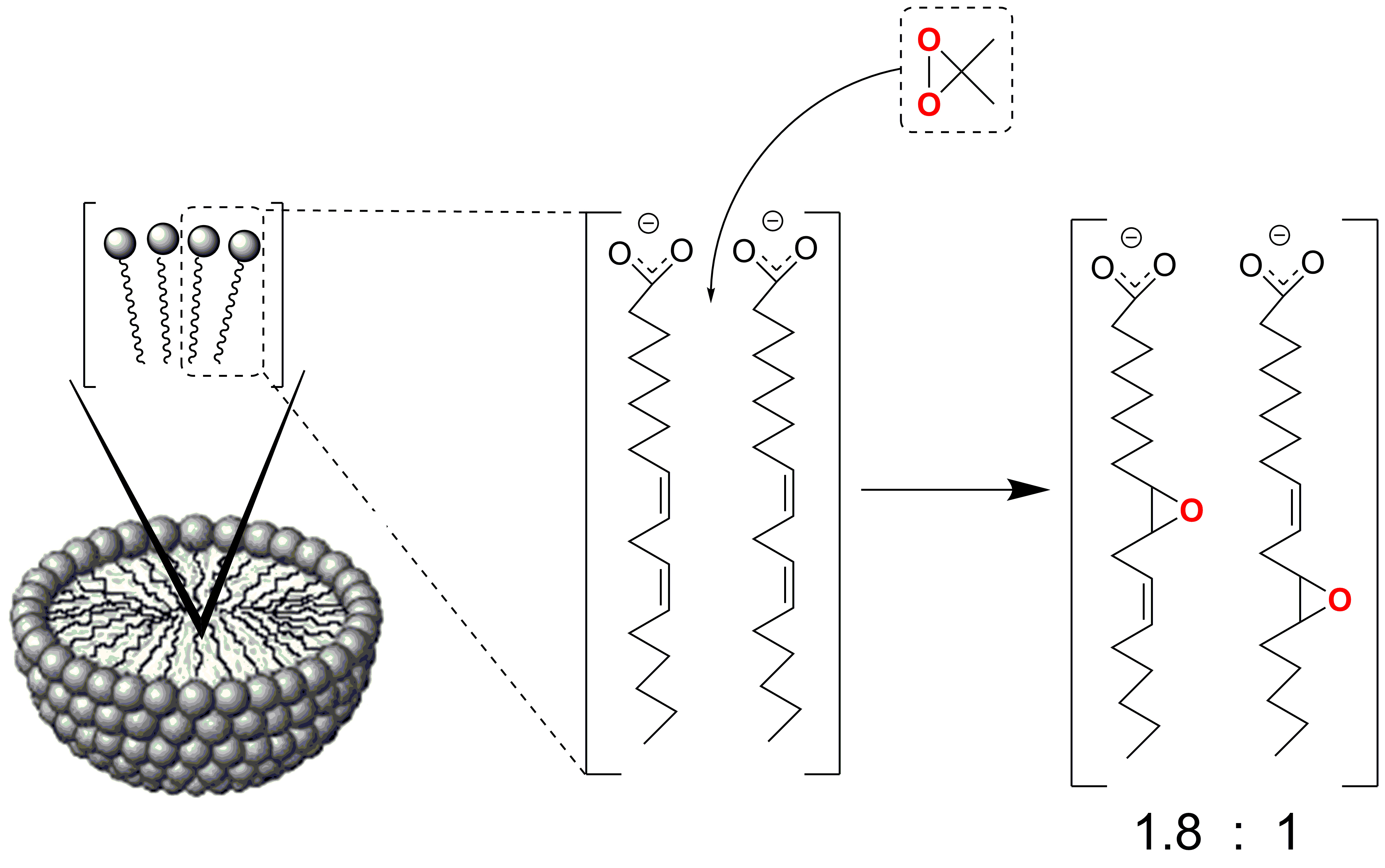
The
use of dimethyldioxirane (DMD) as the epoxidizing agent for polyunsaturated fatty acids was investigated. With fatty acid methyl esters, this is a
convenient method for avoiding acidic conditions, using different solvents, and
simplifying the isolation procedures, with less contamination due to
by-products. The reagent was also tested with free
fatty acids in water. In this case, the supramolecular organization of fatty
acids influenced the reaction outcome, and the epoxidation showed interesting regioselective features. The C=C bonds closest to the
aqueous-micelle interface is the most favored for the interaction with dimethyldioxirane. The preferential epoxidation of linoleic
acid ((9Z,12Z)-octadeca-9,12-dienoic
acid) to the 9,10-monoepoxy derivative was achieved, with a high yield and 65% regioselectivity. In case of arachidonic acid ((5Z,8Z,11Z,14Z)-eicosa-5,8,11,14-tetraenoic
acid) micelles, the regioselective outcome with
formation of the four possible monoepoxy isomers was
studied under different conditions. It resulted to be a convenient synthesis of
‘cis-5,6-epoxyeicosatrienoic acid’ (= 3-[(2Z,5Z,8Z)-tetradeca-2,5,8-trienyl]oxiran-2-butanoic
acid), whereas in reverse micelles, epoxidation mostly gave ‘cis-14,15-epoxyeicosatrienoic
acid ((5Z,8Z,11Z)-13-(3-pentyloxiran-2-yl)trideca-5,8,11-trienoic
acid).
4. A New Synthesis of 5-Hydroxy-6-Methyluracil
(Tetrahedron Letters, DOI: 10.1016/j.tetlet.2012.08.133)
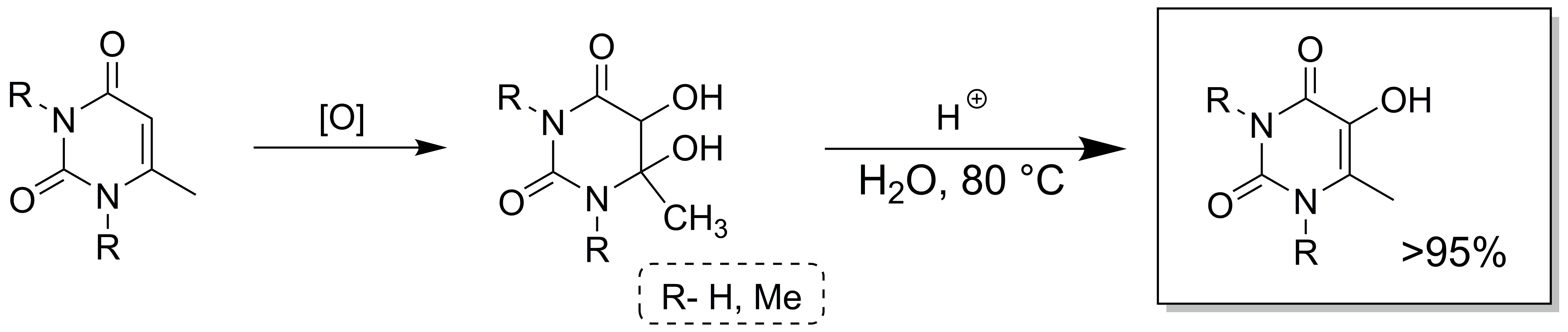
Dehydration
of 5,6-dihydro-5,6-dihydroxy-6-methyl- and
5,6-dihydro-5,6-dihydroxy-1,3,6-trimethyluracil in 0.4 M aqueous sulfuric acid
gives 5-hydroxy-6-methyl- and 5-hydroxy-1,3,6-trimethyluracil in quantitative
yields. Standard DFT calculations at the mPW1k/6-311+G(2df,2pd)//mPW1k/6-31+G(d,p) level have been applied to the question of whether the
dehydration of 5,6-dihydro-5,6-diols of uracil derivatives occurred through a
C5–C6 hydride shift with the loss of water to a protonated
5,6-dihydro-5-oxo-6-methyluracil derivative, or via an E2 reaction. The latter
process was shown to proceed at a substantially lower
activation enthalpy and Gibbs free energy than the route through concerted
migration of a hydride.
5. Oxidation of Highly Strained Cage
Hydrocarbons by Ozone
(Rus. J. Org. Chem., DOI: 10.1134/S1070428015120076)
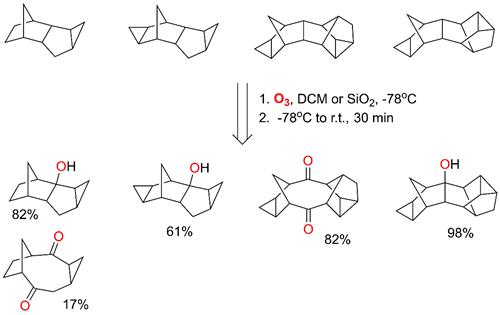
Optimal
conditions for the efficient ozonation of cyclic hydrocarbons
have been identified. The reaction primarily affects
the weakest C–H bond, leading to the intermediate formation of trioxidanes. In certain cases, ozonation
causes cleavage of C–C bonds, resulting in the formation of previously unknown diketones and expanding the scope of oxidative
transformations in strained hydrocarbon systems.